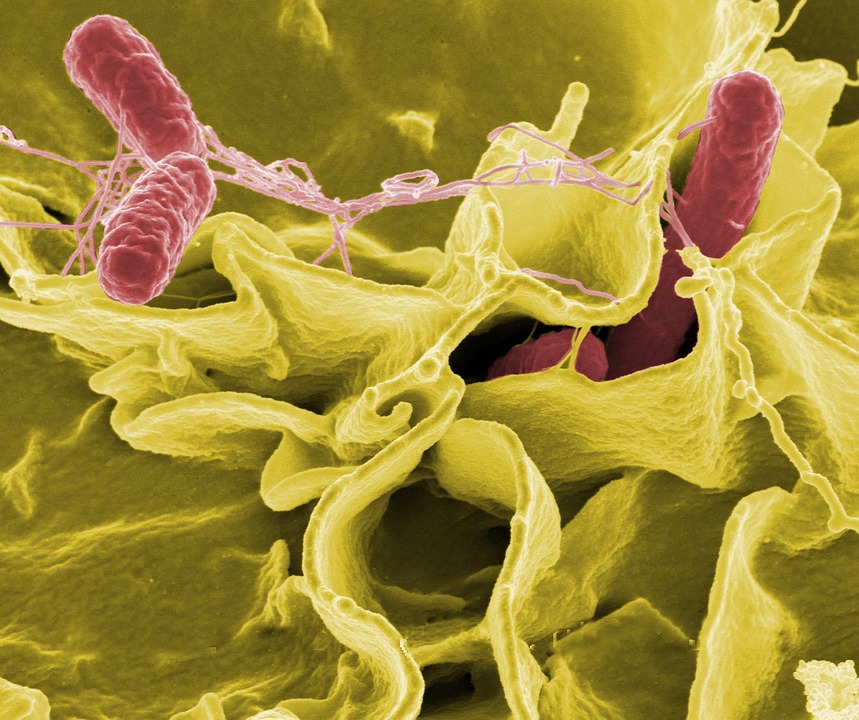
Produce such as lettuce and spinach is routinely tested for foodborne pathogenic bacteria like salmonella, listeria monocytogenes and pathogenic types of E. coli in an effort to protect consumers from getting sick.
Rapid testing of foods may occur, but it still takes time to figure out who is sick and from where the contaminated product originated. That’s far too late for the many Americans who already ate the produce. The current solution, often a multi-state recall, then becomes damage control.
University of Delaware researchers want to spot these bacteria before anyone ever falls ill. As detailed in an article published in the Journal of Food Safety, UD and Delaware-based startup Biospection are about to speed up testing — a lot. Faculty members Harsh Bais and Kali Kniel, alongside former graduate student Nick Johnson, teamed up with Andy Ragone of Biospection to detect foodborne pathogens in three to six hours.
A microbiologist by trade, Kniel is an expert on crossover pathogens like salmonella, which gleefully jump to new hosts like that delicious, fresh lettuce.
“While the produce industry is working diligently to reduce risks associated with microbial contamination, tools like this have incredible potential to improve risk reduction strategies,” said Kniel, professor of microbial food safety who works regularly with industry and government agencies to reduce risk of foodborne illness. “Collaborations like ours between academics and biotechnology companies can enhance technology and impact food safety and public health.”
These pathogens easily find their way into plants, which are unfortunately very welcoming hosts — hosts that can’t tell you where their guests are.
Just like humans, plants use defense mechanisms to fight disease. But some human-borne pathogens learned to push open a plant’s open-entry gates called stomates — pores in the leaves or stem — and make themselves at home.
“Because these bacteria are not true pathogens for plants, you cannot physically see early signs that the plant is under stress,” said Bais, UD professor of plant biology. “Biospection’s technology allows us to say, very quickly, if the opportunistic human pathogen is present in the plant.”
As a chemical physicist working in Wilmington, Ragone got to know Kniel and Bais through Delaware’s scientific community and lab equipment sharing. A relationship built over time, culminating when Kniel, Bais and Ragone applied for and received research funding from a Delaware Biotechnology Institute Center for Advanced Technology (CAT) grant for scientific technology and intellectual property.
The researchers married their interdisciplinary expertise to reduce the risk of foodborne illness, a task that industry and academic researchers struggled with for many years. The result? The team created a multi-spectral imaging platform to look at plant sentinel response. A goal is to use this technique directly on a conveyor, scanning your lettuce before it ever heads to the grocery store.
So how do you see a symptom that you can’t see? The researchers’ technique scans leaves via multispectral imaging and deep UV sensing when the plant is attracting these pathogens. When the researchers looked at benign bacteria, they observed little change. But, with harmful, human-borne pathogens, the test can spot differences in the plant under attack.
“Using listeria as an example, in three to six hours, we see a sharp drop of chlorophyll pigments,” Bais said. “That’s a strong signal that the plant is responding physiologically — a marker of unusual bacteria.”
The new, multi-spectral imaging technique is non-invasive, and lightning fast compared to current tests, where a lab scientist extracts a leaf, grinds it up, plates the bacteria and looks for disease. The current method is not commercially available, but Biospection was awarded a National Science Foundation Small Business Innovation Research grant in 2022 to develop and commercialize it into a real time imaging sensor to inspect plants for disease and other stresses.
“Harsh and Kali were certainly instrumental in the techniques that we developed with multi-spectral imaging and the use of deep ultraviolet fluorescence,” said Ragone, founder and chief technology officer of Biospection. “We built a portable instrument that could be commercialized.”
Vertical farming is an agricultural sector that stands to reap the benefits of this new technology. Using less water and less space, vertical farms are a vital step towards more sustainable agriculture. But when it comes to disease, these farms are just as vulnerable as traditional, outdoor agriculture. An incidence of E. coli means a vertical farm must throw away an entire harvest.
Biospection is already working with agricultural companies to embed the imaging sensor into vertical farms’ shelves and, for outdoor farms, crop drones.
“Working with UD, we’ve laid the scientific foundation to create better instruments,” Ragone said. “We’re working toward an instrument that’s portable, automated and can give an answer in a matter of seconds.”
For future research, Bais has his eye on determining if this technology can differentiate between different microbes.
“If the sentinel response is different from one microbe to the other, that gives us the identity of the microbe based on plant sentinel response. We haven’t gone there yet, but that would be the ultimate achievement,” Bais said. “In one sentinel, then you could differentiate between what benign and harmful microbes does this in terms of one sentinel.”