
Ursolic Acid is a supplement which might aid myelin regrowth and repair in multiple sclerosis.
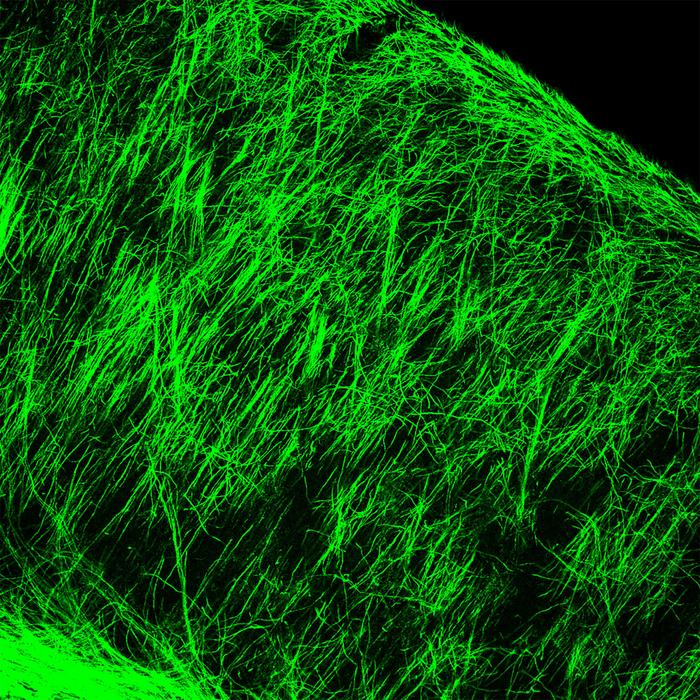
Credit Cincinnati Children’s – Small molecule shows early-stage promise for repairing myelin sheath damage.
When treated with a novel protein function inhibitor called ESI1, mice that mimic the symptoms of multiple sclerosis (MS) and lab-prepared human brain cells both demonstrated the ability to regenerate vital myelin coatings that protect healthy axon function.
This breakthrough appears to overcome difficulties that have long frustrated previous attempts to reverse a form of nerve damage that robs people with MS of motor control and gradually blunts cognitive functions for many people as they age.
“Currently, there are no effective therapies to reverse myelin damage in devastating demyelinating diseases such as MS,” says a top brain research expert at Cincinnati Children’s. “These findings are significant as they offer new pathways for treatment that potentially shift the therapeutic focus from just managing symptoms to actively promoting repair and regeneration of myelin.”
Promoting healing by clearing a roadblock
A critical insight driving the new findings was observing that brain regions damaged by MS still possessed a type of cell needed to repair myelin damage, but the disease activates other cell types and signals that combine forces to silence the repair function.
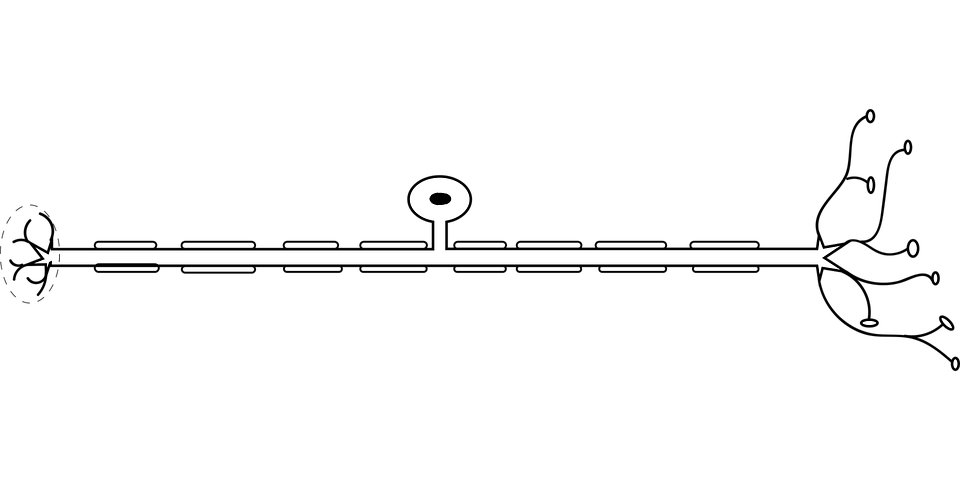
These useful cells in the brain, called oligodendrocytes, are responsible for producing myelin sheaths that wrap around cable-like parts of nerve cells called axons, much like the plastic insulation around a wire. When the protective myelin gets damaged by disease or the wear and tear of age, nerve signalling gets disrupted. Depending on where the damaged nerves lead, the disruptions can affect movement, vision, thinking and so on.
Essentially, the research team found a way to unsilence the silenced repair process, setting the oligodendrocytes (OLs) free to do their jobs.
Pinning down the genetic changes and signals involved in the repair silencing process and finding a small molecule compound that can reverse the silencing was a complex undertaking. The project, which spanned over five years, involved four co-first authors and 29 contributing co-authors from Cincinnati Children’s, the University of Cincinnati, and 14 other institutions, including universities in Australia, China, Germany, India, Singapore, and the United Kingdom.
Among the team’s key findings:
Identifying the mechanism preventing myelin production in MS
Analysis of stored autopsy tissues revealed that OLs within MS lesions lacked an activating histone mark called H3K27ac while expressing high levels of two other repressive histone marks, H3K27me3 and H3K9me3, associated with silencing gene activity.
Finding a compound that can reverse the silencing
The research team scoured a library of hundreds of small molecules known to target enzymes that could modify gene expression and influence the silenced OLs. The team determined that the compound ESI1 (epigenetic-silencing-inhibitor-1) was nearly five times more powerful than any other compounds they considered.
The compound tripled the desired H3K27ac histone mark levels in OLs while sharply reducing levels of the two repressive histone marks. Additionally, the research reveals a new way ESI1 promotes the creation of special membrane-less regulatory hubs known as “biomolecular condensates” within the cell nucleus that control fat and cholesterol levels. These hubs act as central points to boost the production of essential fats and cholesterol needed to make myelin, a crucial component of nerve fibres.
Demonstrating benefits in mice and lab-grown human tissue
In both aging mice and mice mimicking MS, the ESI1 treatment prompted myelin sheath production and improved lost neurological function. Testing included tracking gene activation, measuring the microscopic new myelin sheaths surrounding axons, and observing that treated mice were quicker at navigating a water maze.
Then the team tested the treatment on lab-grown human brain cells. The team used a type of brain organoid, myelin organoids, that is far more simplified than a full brain but still produces complex myelinating cells. When the organoids were exposed to ESI1, the treatment extended the myelin sheath of myelinating cells, the study reports.
Implications and next steps
MS is the most common and best known of several major neurodegenerative diseases. The new findings may spark a new approach to stopping the degenerative effects of these conditions, Lu says.
Myelin regeneration treatment also could be helpful for people recovering from brain and spinal cord injuries.
But the most far-reaching implication of the study is the possibility of using ESI1, or similar compounds, to help slow or even reverse cognitive losses that often occur during aging. Many studies have shown that myelin loss plays a role in age-related loss of cognitive function, Lu says.
However, more research is needed to determine whether human clinical trials can be launched to evaluate ESI1 as a potential treatment. For example, the effects of ESI1 may need to be modulated by adjusting the dose, treatment duration, or using “pulsed therapy” during specific time windows. More study also is needed to determine whether even more effective compounds than ESI1 might be designed from scratch.
“This study is a beginning,” Lu says. “Prior to finding ESI1, most scientists believed that remyelination failure in MS was due to the stalled development of precursors. Now we show a proof of concept that reversing the silencing activity in OLs present in the damaged brain can enable myelin regeneration.”
Research could be important in treating, preventing progression of multiple sclerosis, other neurodegenerative diseases
A compound developed at Oregon Health & Science University appears to protect nerve fibers and the fatty sheath, called myelin, that covers nerve cells in the brain and spinal cord.
The discovery, published in the Journal of Neuroimmunology, could be important in treating or preventing the progression of multiple sclerosis and other central nervous system disorders. The new research in a mouse model advances earlier work to develop the compound – known as sobetirome – that has already showed promise in stimulating the repair of myelin.
“Sobetirome and related drugs are effective at stimulating myelin repair after damage has occurred. Our new findings now suggest that these drugs could also prove to be beneficial for preventing damage from occurring,” said senior author Dennis Bourdette, M.D., former chair and professor emeritus of neurology in the OHSU School of Medicine. “It means that these drugs have a dual effect that we didn’t know about before.”
Nerve fibers carry electrical impulses between nerve cells, and myelin is an insulation-like protective sheath covering nerve fibers.
Myelin and nerve fibers become damaged in multiple sclerosis, slowing or blocking electrical signals required for us to see, move our muscles, feel sensations and think. Researchers previously developed sobetirome as a compound that mimics the effect of the thyroid hormone in stimulating the maturation of precursor cells known as oligodendrocytes, which generate myelin. OHSU scientists developed a strategy to greatly increase the delivery of sobetirome into the brain of mice – remyelinating nerve fiber sheaths after damage had occurred.
The OHSU technology related to these findings is licensed to a startup biotechnology company committed to developing new medications for demyelinating diseases such as MS. Co-founders of the company include Bourdette along with two other co-authors on the new study: Tom Scanlan, Ph.D., professor of physiology and pharmacology in the OHSU School of Medicine, and Ben Emery, Ph.D., associate professor of neurology in the OHSU School of Medicine.
In the new research, scientists tested the compound by inducing an autoimmune disease in a mouse model of MS, causing inflammation damage to myelin and nerve fibers.
Lead author Priya Chaudhary, Ph.D., assistant professor of neurology in the OHSU School of Medicine who is focused on developing therapies for neurodegenerative diseases, said that the technique is a common step in drug discovery.
“It is important to show the effectiveness of potential drugs in a model that is most commonly used for developing new therapies,” Chaudhary said.
The researchers discovered that they were able to prevent damage to myelin and nerve fibers from occurring, by stimulating a protective response in the cells that make and maintain myelin. They also reduced the activity of migroglia, a type of inflammatory cell in the brain and spinal cord that’s involved in causing damage in multiple sclerosis and other diseases.
“The effects are impressive and are at least in part consistent with a neuroprotective effect with particular inhibition of myelin and axon degeneration, and oligodendrocyte loss,” the authors write.
The discovery, if proven in clinical trials involving people, could be especially useful for people who are diagnosed with multiple sclerosis early in the disease’s progression.
“The drug could protect the nervous system from damage and reduce the severity of the disease,” Bourdette said.
A scientific breakthrough provides new hope for millions of people living with multiple sclerosis. Researchers at Oregon Health & Science University have developed a compound that stimulates repair of the protective sheath that covers nerve cells in the brain and spinal cord.
The discovery, involving mice genetically engineered to mimic multiple sclerosis, published in the journal JCI Insight.
MS is a chronic condition that affects an estimated 2.3 million people worldwide. In MS, the sheath covering nerve fibers in the brain and spinal cord becomes damaged, slowing or blocking electrical signals from reaching the eyes, muscles and other parts of the body. This sheath is called myelin. Although myelin can regrow through exposure to thyroid hormones, researchers have not pursued thyroid hormone therapies due to unacceptable side effects.
Although several treatments and medications alleviate the symptoms of MS, there is no cure.
“There are no drugs available today that will re-myelinate the de-myelinated axons and nerve fibers, and ours does that,” said senior author Tom Scanlan, Ph.D., professor of physiology and pharmacology in the OHSU School of Medicine.
Co-author Dennis Bourdette, M.D., chair of neurology in the OHSU School of Medicine and director of the OHSU Multiple Sclerosis Center, said he expects it will be a few years before the compound advances to the stage of a clinical trial involving people. Yet the study provides fresh hope for patients in Oregon and beyond.
“It could have a significant impact on patients debilitated by MS,” Bourdette said.
The discovery, if ultimately proven in clinical trials involving people, appears to accomplish two important goals:
“We’re taking advantage of the endogenous ability of thyroid hormone to repair myelin without the side effects,” said lead author Meredith Hartley, Ph.D., an OHSU postdoctoral researcher in physiology and pharmacology.
Co-authors credited the breakthrough to a collaboration that involved scientists and physicians with expertise ranging across neurology, genetics, advanced imaging, physiology and pharmacology.
One patient said the research could be a “total game-changer” for people with MS.
Laura Wieden, 48, has lived with multiple sclerosis since being diagnosed in 1995. The daughter of Portland advertising executive Dan Wieden, she is the namesake and board member of the Laura Fund for Innovation in Multiple Sclerosis, which funded much of the research involved in the study published today.
“I am really optimistic,” Wieden said. “I hope that this will be literally a missing link that could just change the lives of people with MS.”
Scanlan originally developed sobetirome as a synthetic molecule more than two decades ago, initially with an eye toward using it to lower cholesterol. In recent years, Scanlan’s lab adapted it as a promising treatment for a rare metabolic disease called adrenoleukodystrophy, or ALD.
Six years ago, Bourdette suggested trying the compound to repair myelin in MS.
Supported by funding provided through the Laura Fund and the National Multiple Sclerosis Society, the team turned to Ben Emery, Ph.D., an associate professor of neurology in the OHSU School of Medicine. Emery, an expert who previously established his own lab in Australia focused on the molecular basis of myelination, genetically engineered a mouse model to test the treatment.
With promising early results, researchers wanted to see if they could increase the amount of sobetirome that penetrated into the central nervous system.
They did so through a clever trick of chemistry known as a prodrug strategy.
Scientists added a chemical tag to the original sobetirome molecule, creating an inert compound called Sob-AM2. The tag’s main purpose is to eliminate a negative charge that prevents sobetirome from efficiently penetrating the blood-brain barrier. Once Sob-AM2 slips past the barrier and reaches the brain, it encounters a particular type of brain enzyme that cleaves the tag and converts Sob-AM2 back into sobetirome.
“It’s a Trojan horse type of thing,” Scanlan said.
Researchers found that the treatment in mice not only triggered myelin repair, but they also measured substantial motor improvements in mice treated with the compound.
“The mouse showed close to a full recovery,” Scanlan said.
Scientists say they are confident that the compound will translate from mice to people. To that end, OHSU has licensed the technology to Llama Therapeutics Inc., a biotechnology company in San Carlos, California. Llama is working to advance these molecules toward human clinical trials in MS and other diseases.
Bourdette said even though it may not help his patients today, he’s optimistic the discovery eventually will move from the lab into the clinic.
“Right now, what it means is hope,” he said.

Together with his team Prof. Mikael Simons researches the formation and removal of the myelin sheathes which surround nerve fibers and which are destroyed in Multiple Sclerosis. A. Eckert / TU
Multiple Sclerosis (MS) is a chronic inflammatory disease of the central nervous system, in which the body’s own immune cells attack the fatty, insulating myelin sheath surrounding nerve fibers. The regeneration of intact myelin sheaths is a necessary prerequisite for patients to recover from MS relapses. Nevertheless, the body’s ability to regenerate myelin decreases with age.
A team led by Prof. Mikael Simons from the Technical University of Munich (TUM) has now published a possible explanation in the journal Science: Fat derived from myelin, which is not carried away rapidly enough by phagocytes can trigger chronic inflammation that in turn impedes regeneration. Furthermore, in a second publication Simons’ team describes the discovery of novel cell type, which appears only when a myelin sheath is being created.
The myelin sheath plays a decisive role in the function of the central nervous system: it is a specialized membrane enriched in lipids, which insulates nerve fibers so that electrical signals can be passed on quickly and efficiently. In MS, there is a multifocal autoimmune attack against the myelin sheath in the central nervous system, which causes neurological deficits such as loss of motor function. Regeneration of myelin is possible, but in MS it is inadequate.
One of the reasons is presumably chronic inflammation occurring in the lesions. A team led by TUM Molecular Neurobiology professor Mikael Simons has now discovered that after the destruction of myelin crystalline cholesterol can trigger persistent inflammation which prevents regeneration, similar as in arteriosclerosis.
Dangerous crystals
“Myelin contains a very high amount of cholesterol,” explains Prof. Simons. “When myelin is destroyed, the cholesterol released has to be removed from the tissue.” This is performed by microglia and macrophages, also referred to as phagocytes. They take up the damaged myelin, digest it and transport the non-digestible remainder, such as cholesterol, out of the cell by transport molecules. However, if too much cholesterol accumulates in the cell, cholesterol can forms needle-shaped crystals, which cause damage the cell. Using a mouse model, Simons and his team showed the devastating impact of the crystalline cholesterol: It activates the so-called inflammasome in phagocytes, which results in the release of inflammatory mediators, attracting even more immune cells. “Very similar problems occur in arteriosclerosis, however not in the brain tissue, but in blood vessels,” says Simons.
How well the microglia and macrophages did their job was ultimately also dependent on the age of the animal: the older the animal, the less effective was the clearance of cholesterol and the stronger the chronic inflammations. “When we treated the animals with a medication that facilitates the transport of cholesterol out of the cells, inflammation decreased and myelin was regenerated,” says Mikael Simons. Next he and his team want to investigate whether this mechanism can be used therapeutically to promote regeneration in MS.
Newly discovered cells indicate regeneration
A crucial prerequisite for the development of therapies that promote repair is a better understanding of myelin formation. In another study, recently published in the journal Science Translational Medicine and led by Prof. Simons and Prof. Christine Stadelmann of the University of Göttingen’s Institute of Neuropathology, provides important new insights into this process. The scientists discovered a novel oligodendroglial cell type. Oligodendrocytes are specialized glial cells that are responsible for myelination in the central nervous system.
“We believe that the BCAS1-positive oligodendrocytes that we discovered represent an intermediate stage in the development of myelin-forming cells. In humans they can only be identified for a relatively short period of time, exactly then when myelin is actually being formed,” says Mikael Simons. In the human brain, for example, they are found in newborns, which generate myelin at high rate. In adults, these cells disappear, but they can be re-formed when myelin has been damaged and needs to be regenerated.
“We hope that the BCAS1 positive cells will help us to identify new regenerative medicines,” says Mikael Simons. We can now rapidly screen for drugs that promote the formation of these cells, he adds. Furthermore they could be used to get a better understanding of exactly when and how myelin is created during the course of a human life, he says.