Here are the best ten exercises for rheumatologist arthritis patients. Together with Dr Melissa...
Rheumatoid arthritis
If you have friends or family members with Rheumatoid arthritis, you might want to...
Shown above are the representative tracks of H3K27ac ChIP-seq at NFATc1, PRDM1, and MYC...
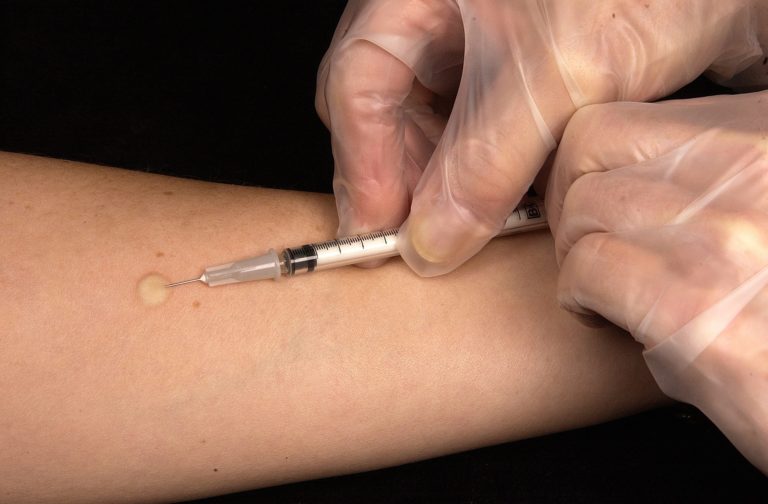
For chronic conditions such as rheumatoid arthritis, treatment often involves lifelong injections. Fear of...
With the GreenFare 21 Day Kickstart program, an easy path to reversing most chronic...