
In this video, I explore the connection between autism and mental health challenges.

Most persons living with multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS) continue to get worse. Researchers and healthcare providers don’t know why or how to stop it. Although the two diseases are different, they share the common feature of nerve cell death in the brain and spinal cord. Our research team has discovered how nerve cells like these die and designed new drugs to stop cell death and encourage regeneration! By stopping nerve cell death and promoting their recovery, we believe we can stop MS and ALS in their tracks, improving the lives of persons living with these devastating diseases. Dr. Michael C. Levin (MD) is the Saskatchewan Multiple Sclerosis (MS) Clinical Research Chair and Professor of Neurology at the University of Saskatchewan (USask) College of Medicine. An MS specialist and neuroscientist, Levin has been caring for persons living with MS and conducting research into the cause and treatments for MS for most of his career.
At the University of Tennessee Health Science Center, he was professor, Chief of the Neurology Service at the Memphis Veterans Affairs Medical Center, leading the MS clinic and performed research on RNA binding proteins in MS. His work has been published in top journals, including The New England Journal of Medicine, Nature Medicine, Annals of Neurology, Glia, and Journal of Neuroscience Research. Dr. Levin and his team have received more than 100 awards for academic excellence. He is editor of the Neurology Section of the Merck Manual, on the Medical Advisory Committee of MS Canada and honoured as one of the ‘Best Doctors in America’.

Poor diet continues to take a toll on American adults. It’s a major risk factor for obesity, type 2 diabetes, cardiovascular disease, and certain cancers, and more than one million Americans die every year from diet-related diseases, according to the Food and Drug Administration. Poor diet and food insecurity are also costly, attributing to an estimated $1.1 trillion in healthcare expenditures and lost productivity. These burdens also contribute to major health disparities by income, education, zip code, race, and ethnicity.
In a study from the Food is Medicine Institute at the Friedman School of Nutrition Science and Policy at Tufts University, researchers found that diet quality among U.S. adults improved modestly between 1999 and 2020. However, they also found that the number of Americans with poor diet quality remains stubbornly high. Most notably, disparities persist and, in some cases, are worsening.
“While we’ve seen some modest improvement in American diets in the last two decades, those improvements are not reaching everyone, and many Americans are eating worse,” says Dariush Mozaffarian, cardiologist and director of the Food is Medicine Institute and senior author on the study. “Our new research shows that the nation can’t achieve nutritional and health equity until we address the barriers many Americans face when accessing and eating nourishing food.”
The study investigated data from 10 cycles of the National Health and Nutrition Examination Survey between 1999 and 2020. This nationally representative survey includes repeated 24-hour dietary recalls, where people report all foods and beverages consumed during the prior day. The study analyzed 51,703 adults who completed at least one valid 24-hour recall, with 72.6% having done two recalls.
Diet quality was measured using the American Heart Association diet score, a validated measure of a healthy diet that includes components like fruits, vegetables, beans and nuts, whole grains, sugary beverages, and processed meat. Researchers found that the proportion of adults with poor dietary quality decreased from 48.8% to 36.7% over these two decades, while those with intermediate diet quality increased from 50.6% to 61.1%. They also found that the proportion of adults with an ideal diet improved but remained starkly low, from 0.66% to 1.58%.
Specific changes contributed to these trends, including higher intakes of nuts/seeds, whole grains, poultry, cheese and eggs. Researchers also found lower consumption of refined grains, drinks with added sugar, fruit juice and milk. Total intake of fruits and vegetables, fish/shellfish, processed meat, potassium, and sodium remained relatively stable.
When the analysis focused on key subgroups, the researchers found that these improvements were not universal. Gains in dietary quality were highest among younger adults, women, Hispanic adults, and people with higher levels of education, income, food security, and access to private health insurance. They were lower among older adults, men, Black adults, and people with lower education, less income, food insecurity, or non-private health insurance. For example, the proportion of adults with poor diet quality decreased from 51.8% to 47.3% among individuals with lower income, decreased from 50.0% to 43.0% among individuals with middle income, and decreased from 45.7% to 29.9% among individuals with higher income.
“While some improvement, especially lower consumption of added sugar and fruit drinks, is encouraging to see, we still have a long way to go, especially for people from marginalized communities and backgrounds,” adds first author Junxiu Liu, a postdoctoral scholar at the Friedman School at the time of the study, now assistant professor at the Icahn School of Medicine at Mount Sinai.
“We face a national nutrition crisis, with continuing climbing rates of obesity and type 2 diabetes,” Mozaffarian said. “These diseases afflict all Americans, but especially those who are socioeconomically and geographically vulnerable. We must address nutrition security and other social determinants of health including housing, transportation, fair wages, and structural racism to address the human and economic costs of poor diets.”
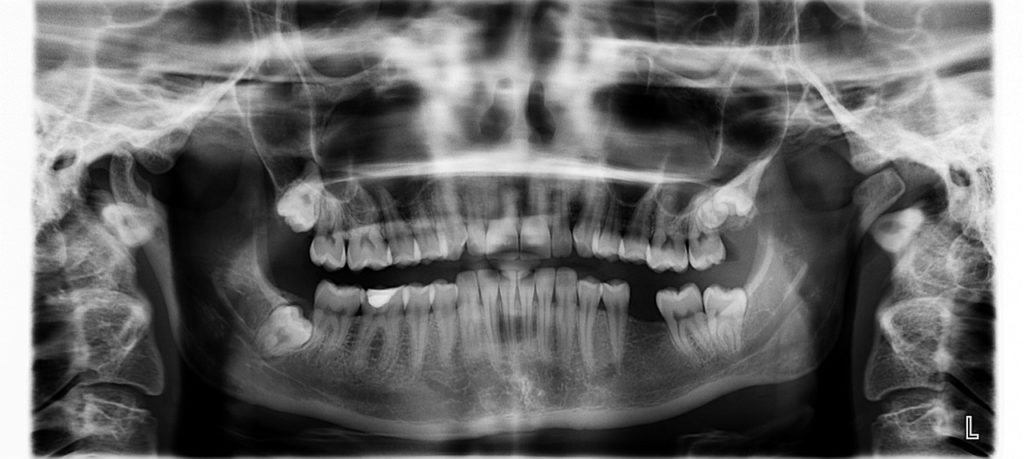
Improvements in dental care, more people living longer and the social value placed on having a healthy smile has led to people keeping their own teeth longer, but it has also led to an increasing number of people needing some kind of restoration work including crowns, bridges and implants.
Many of these treatments remain unobtainable for most people due to the availability of NHS dentists and the high cost of private dental work. Removable dentures are often the only viable option for anyone experiencing tooth loss, with an estimated 10 to 15 per cent of the population wearing them.
A new study by researchers at the University of Sheffield’s Healthy Life Span Institute and the School of Clinical Dentistry has highlighted the emotional struggles and hidden challenges patients experience when having dentures fitted. This is the first study to map out the patient journey and how this experience can affect the overall success of the treatment.
The study found that patients think about their denture journey in four stages:
These feelings and how dentists understand and manage them can influence the patient outcomes. A dentist’s empathy during this adjustment period is crucial for successful denture use and better patient outcomes.
The study also identified that wearing removable dentures can be a hidden disability for many. People with dentures feel they have to hide them due to feeling embarrassed or worrying they will fall out. Some patients also avoided social situations
Lead researcher Barry Gibson, Professor of Medical Sociology at the University of Sheffield, said, “Tooth loss can be hugely traumatic, and this study has uncovered just how challenging it is for people needing partial dentures. Feelings such as embarrassment or shame can significantly affect the process of having dentures made and fitted. On top of this if they don’t fit properly this can make everyday activities such as speaking, eating and drinking very difficult which affects a person’s quality of life. The impact can be so dramatic that it can impact their confidence to leave the house. This can have a devastating and lasting impact.
“Understanding the emotional difficulties identified in the study will help dentists to improve the care given to denture patients and lead to a more successful and better experience for everyone.”
Dr. Shizuka Shimabukuro, lead author of the study, explains a game that is included in the program, which mothers can play with their children to improve their visual memory while having fun. CREDIT Okinawa Institute of Science and Technology
Attention deficit hyperactivity disorder (ADHD) is a common neurodevelopmental disorder characterized by elevated levels of inattention, impulsivity, and hyperactivity that can impair academic and social functioning. ADHD is also associated with increased levels of parenting stress and less effective parenting practices and can disrupt the parent-child relationship. The importance of support for parents of children with ADHD is widely acknowledged in Japan, but specialized parent training programs targeting ADHD have not been available.
However, a new program developed at the Okinawa Institute of Science and Technology (OIST) aims to reduce the strain on families of children with ADHD by helping mothers improve their ADHD-specific parenting skills, as well as developing confidence in their own parenting. Dr Shizuka Shimabukuro has worked to develop and evaluate Well Parent Japan (WPJ). WPJ is a 13-week group-based parent training program that enhances mothers’ psychological well-being and teaches culturally tailored parenting skills for ADHD. She is the lead author of a recently published paper in the Journal of Child Psychology and Psychiatry (DOI) that evaluates the efficacy and cost-effectiveness of WPJ compared with treatment as usual for Japanese mothers of children with ADHD.
“We implemented WPJ across three sites in Japan and found that the program was more effective than treatment as usual in these settings, as well as being moderate in cost to deliver,” explains Dr. Shimabukuro, and adds that, importantly, “the study was conducted in regular hospitals and a developmental support centre, not the research lab, as we wanted to test if it worked when delivered in the community.”
Finding solace in the community
The group-based approach was found to be very effective in providing social support and encouraging shared learning amongst the participating mothers, who might otherwise feel isolated or hesitate to seek help in dealing with their children’s difficulties. “The primary caregiver in Japan is usually the mother, and because they hesitate to put their own needs ahead of others, they often feel alone with their problems. We wanted to invite them to a comfortable, shared space where everyone is dealing with similar issues and can safely share their struggles and concerns and learn from and support one another,” explains Dr. Shimabukuro.
Just as the mothers came together to share and address their parenting difficulties, so is WPJ the product of ten years of co-production and collaboration between researchers, clinicians, parents, and children with ADHD, drawing on international literature and the voices of Japanese parents who participated in the studies. Professor Gail Tripp, head of the unit and another author on the paper, describes the present study as an exercise in crossing divides: “We worked closely with a local hospital here in Okinawa, two university hospitals in other parts of Japan, an economist in Tokyo, and a research colleague in the UK. OIST Innovation supported the study and our efforts to move toward broader implementation. It’s a highly collaborative effort, and I’m glad to see it coming to fruition.”
From lockdown to the future
The process of testing the efficacy of WPJ was not without its challenges. The clinical trials began just before the onset of the unexpected coronavirus pandemic, which meant they had to adapt the original research design to the changing social conditions. “We were trying to coordinate the trials across three sites, with the regulations constantly changing with the nature of the pandemic,” as Dr. Shimabukuro recounts it. “I’m very grateful and proud of the work that the research group leaders put into first and foremost ensuring the health and safety of our research participants, while also ensuring robust data.”
Despite the difficulties faced, the researchers were pleased to see that WPJ was effective in ‘the real world’, with the program outperforming treatment as usual in reducing parenting-specific stress, improving parenting efficacy and reducing family strain. “Considering the disruptions brought by COVID, we had an extraordinarily high participant retention,” explains Prof. Tripp, adding that “comparable studies from abroad in non-pandemic conditions usually report a participant attrition rate – the rate of participants of dropping off during the study – of about 15%, but ours was just around 7%.” Dr. Shimabukuro suggests that “our results show how much parents engaged with the program and valued the time and space to talk about their children, even during the height of the COVID crisis.”
The high participant retention and the positive results confirms the value of programs like the WPJ in Japan. As Dr. Shimabukuro puts it, “as parents are agents of change for their children, it is very important to take care of them before they can take care of others.”
Dr. Shimabukuro is now turning her focus to another environment where children with ADHD spend much of their time – schools. “Teachers are also important agents of change for the children, and the children spend so much of their time with them – but like parents, they often face the challenges that ADHD can bring alone. It would be much easier for children with ADHD to establish good behavioral habits if parents and teachers dealt with any problems that arise consistently,” explains Dr. Shimabukuro.
The team is now running a feasibility study for implementing an educational video series, a modified version of WPJ, for teachers in schools. Both to educate the teachers about what it means for a child to have ADHD and how to adjust their teaching techniques to accommodate this, but also to – like with the mothers – provide a space for the teachers to share their professional insights and experiences of accommodating children with ADHD, with one another. As licensed clinical psychologists, Dr. Shimabukuro and Prof. Tripp are working to combine their clinical experience with their research in the lab for the good of the children, their families, and their communities. In Dr. Shimabukuro’s words, “we are ultimately hoping to help enhance people’s understanding of ADHD, improve the quality of support and increase the number of places to receive help in the community – we want to help these families under strain.”